Decentralized Clinical Trials: What’s the Vendor Landscape?
Everest Group brings clarity to the decentralized clinical trials vendor landscape.
Decentralized Clinical Trials, or DCTs, are clinical trials in which data is collected through sensors or remote monitoring devices carried by a patient without the need to visit a site. They can deliver many benefits to pharmaceutical companies, including cost savings, better patient recruitment and retention, and improved data quality.
Before the COVID-19 pandemic, while there was significant information on DCT benefits, there were only a few pilots. However, recently decentralized clinical trials have proved to be a saving grace to restart paused clinical trials. Additionally, recent technological advances, the proliferation of wearables, and the FDA’s push for the industry to adopt DCTs following the COVID-19 situation have made the DCT landscape ripe for disruption.
But with things moving so quickly, how do pharmaceutical companies choose a DCT provider?
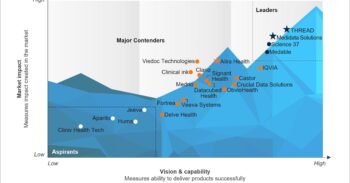
In our Decentralized Clinical Trial Products PEAK Matrix® Assessment 2021, we explore this quickly developing market and assess the capabilities of 15 IT vendors specific to DCT products. The assessment thoroughly details the vendors’ capabilities, potential, and market impact.
Learn more about the emerging decentralized clinical trial landscape through key findings from the assessment and our other research on this topic.