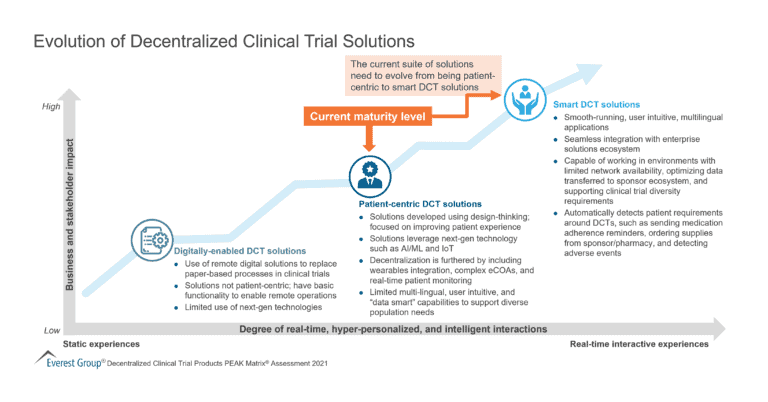
There is now an expectation to showcase a patient-centric platform with one underlying factor – keep it simple for the patient. Some examples of clinical trial optimization solutions for positive patient experience include a simple, user-friendly interface, an easy to setup platform or app, smooth operation that does not malfunction or pester patients, robust education and training, and offering multilingual support.
Enterprises want clinical trial solutions to integrate smoothly into the daily lives of patients. Patients should not feel like a significant amount of digital literacy is required to participate because it might deter them from participating in the trial, making the patient experience an even more important clinical trial solution. The five factors to enhance patient experience are:
• User-friendly interface – The user interface (UI) of DCT applications and devices must be simple, yet effective. They must provide clear instructions and display only relevant and well-organized content
• Easy to set up platform/app – Patients should have an easy time setting up a wearable, sensor, or application. It should be intuitive even to an average user with limited exposure to digital devices
• Smooth operation – The applications or devices should not pester patients with unnecessary notifications, malfunctions, or failures that would cause unwanted frustrations, resulting in reduced patient engagement
• Robust education and training – Patients come with different levels of digital literacy, and they need to be supported during the trials. They must be aware of how to enroll themselves for the trial, schedule appointments, feed in data, and get important information about their health and the trial. Sponsors can create the knowledge pool, conduct training sessions, and build artificial intelligence (AI) bots to provide education and training to patients
• Multilingual app and support – To reach a global audience, multilingual offering and support must be available. The devices or applications used should provide instructions and information in the commonly used languages across the world. If a trial is geographically focused, the regional language should be configured in the device