Clinical trial vendors
Before the COVID-19 pandemic, there was a hefty amount of information on Decentralized Clinical Trial (DCT) benefits, but vendor selection and management in clinical trials remained a difficult problem to solve.
In recent post-pandemic times, decentralized clinical trials have proven to be the key drivers to resume paused clinical trials. Recent technological advances, the production of wearables, and the FDA’s push for the industry to adopt DCTs following the COVID-19 situation have made the DCT landscape ready for disruption as multiple clinical trial vendors that address DCT requirements have recently developed.
The landscape continues to experience heavy fundraising. Co-innovation, continuous product improvements, and market education have allowed clinical trial vendors to focus on increasing trust, speed up trial times, and deliver a better experience in running decentralized clinical trials.
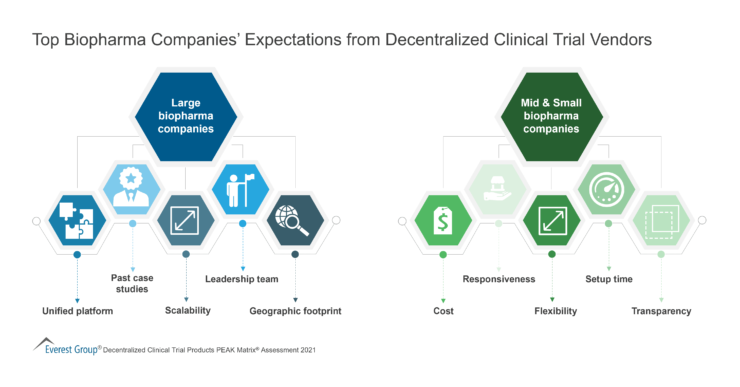
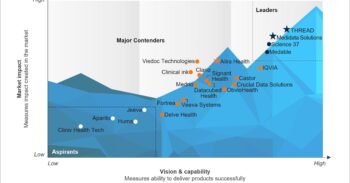
To solve for better vendor selection and management in clinical trials, it is important to remember to weigh clinical trial vendors on several capability and market success-related dimensions. This enables buyers to choose a clinical trial vendor based on their sourcing considerations.