Risk-Based Monitoring (RBM), once seen as a breakthrough in clinical trial oversight, now faces challenges in keeping up with the growing complexity and regulatory demands of modern trials.
While it brought significant improvements in monitoring efficiency, now it is no longer sufficient on its own.
In today’s world of decentralized trials, real-time data flows, and global collaboration, is only “monitoring” enough? The answer is becoming a clear “NO”. Sponsors and regulators now demand much more than just reactive monitoring and isolated site-level checks.
Reach out to discuss this topic in depth.
RBM, with its limited scope and focus on centralized data triggers, was a step forward in its time. However, as the industry innovates, sponsors realized that it lacks the breadth and adaptability needed to support quality across the full clinical trial lifecycle. RBM needed a positive accelerated spin that would not just stop at monitoring; rather cover a broader horizon including aspects of protocol design, patient engagement, and regulatory readiness.
So, what’s next?
To meet the rising demands, the industry is shifting towards Risk-Based Quality Management (RBQM), a truly holistic framework that embeds risk and quality thinking into every phase of a clinical trial, not just during monitoring.
RBQM is a method used in clinical trials to manage and reduce risks by focusing on what really matters. Instead of just monitoring the data from trial sites, it looks at the whole trial process, across trial planning to execution. It also helps identify potential risks earlier, prioritizes the most important aspects of the trial, and ensures that quality is maintained at every stage. The goal of incorporating RBQM in a clinical trial is to improve efficiency, ensure patient safety, and make the trial process smoother by focusing on key risk areas, rather than checking every detail.
Exhibit 1: RBQM vs RBM
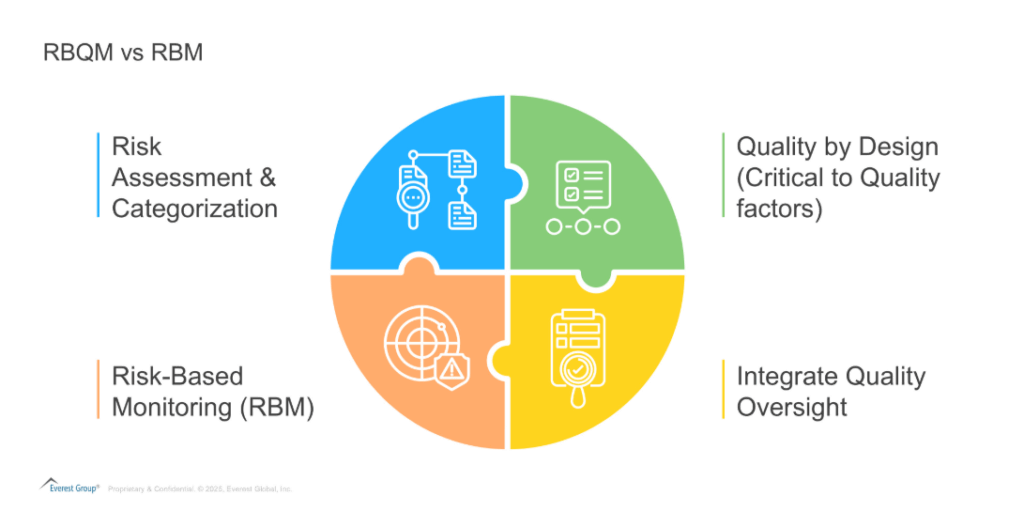
RBQM vs. RBM: A strategic shift from monitoring to total quality management in clinical trials
Identification
RBQM begins far earlier in the trial lifecycle than RBM. It integrates risk assessment into protocol design, site feasibility, and vendor selection, well before any monitoring activities begin. This early identification allows teams to anticipate and mitigate risks before they affect trial conduct or data quality.
Critical-to-Quality (CtQ) focus
RBQM also emphasizes the identification of Critical-to-Quality factors, those trial elements that have a direct impact on patient safety and data integrity. This focus informs study design and operational planning, ensuring resources are directed toward what matters most. RBM, by contrast, lacks this strategic prioritization.
Integrated quality oversight
While RBM is primarily a monitoring function, RBQM embeds quality oversight across the entire organization. It connects clinical operations, data management, quality assurance, and safety teams, creating a continuous feedback loop that supports high-quality outcomes at every stage of the trial.
Adaptive trial execution
RBQM enables dynamic adjustment of trial activities based on ongoing risk reassessment. Unlike RBM, where monitoring plans are often fixed, RBQM allows for flexibility. As new risks emerge or site performance shifts, processes and oversight can be quickly adapted to maintain control and efficiency.
Cross-functional collaboration
RBQM is a collaborative process as it requires coordination between multiple departments, including clinical, regulatory, Quality Assurance (QA), data, and more, to identify risks, implement controls, and manage quality collectively. This cross-functional engagement goes well beyond the scope of RBM, which is often limited to monitoring teams
Regulatory alignment
RBQM aligns directly with the broader intent of ICH E6(R2) and the upcoming R3 guidance, which emphasize quality-by-design, proactive risk assessment, and comprehensive oversight. While RBM aligns with centralized monitoring aspects, it doesn’t meet the full expectations of these evolving regulatory frameworks.
Is RBQM just another layer of oversight, or a smarter way forward?
Let’s break it down.
As trials become more complex and data-rich, traditional monitoring can no longer keep up. RBQM doesn’t just refine existing practices, it redefines them. By embedding risk awareness and quality thinking from day one, RBQM drives efficiency, reduces protocol deviations, and improves regulatory readiness.
The below exhibit showcases how RBQM is transforming critical areas of clinical research:
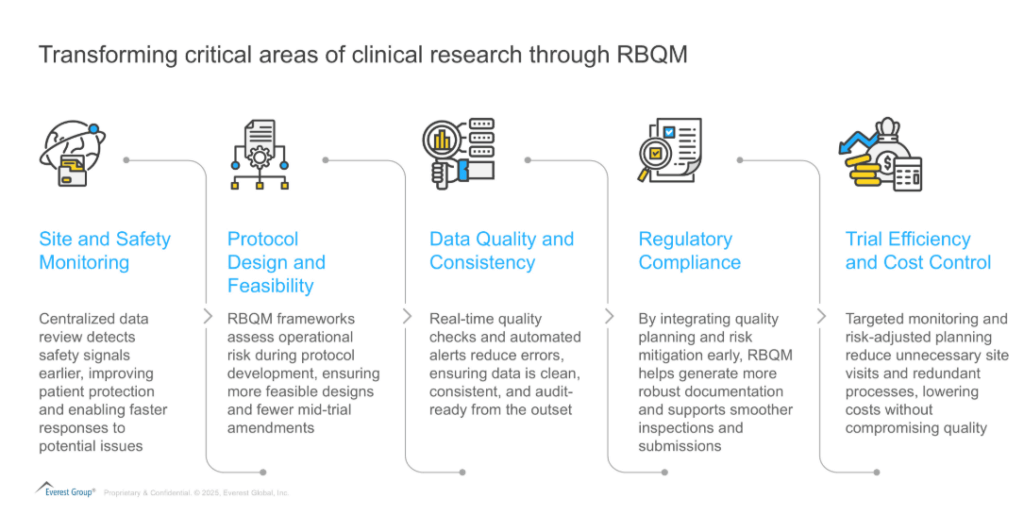
Exhibit 2: Transforming critical areas of clinical research through RBQM
The bottom line?
The adoption of RBQM marks a pivotal shift in how clinical trials are designed, conducted, and overseen. Far from being just a regulatory expectation, RBQM is a strategic enabler, one that enhances efficiency, safeguards patient safety, and improves the reliability of trial outcomes. By embedding quality into every phase of the trial lifecycle, it transforms quality management from a reactive task into a proactive, continuous process.
As the complexity of clinical research grows, so does the need for smarter, more integrated approaches to risk and quality. Traditional models, including RBM alone, are no longer sufficient to meet the evolving demands of regulators, sponsors, and patients alike. RBQM provides the structure and flexibility needed to manage these demands effectively, without compromising on speed, compliance, or data integrity. RBQM isn’t just a better way to monitor, it’s a smarter way to manage.
If you found this blog interesting, check out our Optimizing Clinical Trials With Advanced Data Management Strategies | Blog – Everest Group, which delves deeper into another topic regarding clinical trials.
If you have any questions, are interested in exploring how RBQM is transforming the clinical trials landscape, or would like to discuss these topics in more detail, feel free to reach out to Kavya Murki ([email protected]) and Nisarg Shah ([email protected]).