
How Decentralized Clinical Trials Put the Patient Experience at the Forefront
With the COVID-19 pandemic accelerating the adoption of Decentralized Clinical Trials (DCT), the opportunity to deliver a patient-centric experience is viewed as a top benefit of this alternative mode of clinical trials that uses digital and remote technologies. What factors are enterprise buyers looking for DCT vendors to provide in their platforms to increase satisfaction and ultimately drive patient enrollments? Learn about the five factors that go into a “patient centered” experience in this blog.
When COVID-19 brought traditional clinical trials to an abrupt halt, Decentralized Clinical Trials (DCT) proved to be a savior for sponsors looking to safely restart their paused research activities. While DCTs have been around for a decade and are slowly gaining traction, the pandemic accelerated the use of these alternative methods to collect clinical trial data through sensors or remote monitoring devices carried by a patient.
The top reason for moving toward this model has been its patient-centered focus that makes it easier for more people from a broader geographic area to participate in trials without the need to visit a site.
The growing mainstream acceptance for DCTs has increased the appetite among clinical research organizations (CROs) and sponsors to adopt the latest technologies and virtual models for clinical trials. This has resulted in an uptick in innovation and DCT product adoption recently. We see DCT vendors increasingly focus on co-innovation, continuous product improvement, and market education to help clients get started on their DCT journey.
Top benefits of DCT adoption
Our Decentralized Clinical Trial Products PEAK Matrix® Assessment 2021 found the most promising benefit for enterprises to consider decentralizing their trials is the opportunity to enhance the patient experience – a benefit that two out of three DCT product buyers also agree with based on Everest Group interviews. Other advantages of DCTs include reducing trial costs and timelines, attracting a more diverse patient population, and capturing real-time data for trials.
With DCTs, patients can now take part in a study from the comfort of their homes, spend more time with their family members, and focus on work and other responsibilities. This mode of clinical trial also opens the door to the patients who suffer from mobility issues and allows sponsors to reach a global audience, increasing inclusivity and diversion.
This new patient-centric approach is driving increased enrollment and retention rates. With these valuable benefits, it is not surprising that having a people-orientated platform has become central to enterprise buyers in making their sourcing decisions – even more so than innovation or reviews from other buyers.
What do buyers want from DCT vendors?
What do enterprises buyers mean when they talk about patient experience? Multiple facets contribute to the notion of patient experience as presented in the exhibit below.
Exhibit 1: What enterprises buyers mean when they say patient experience
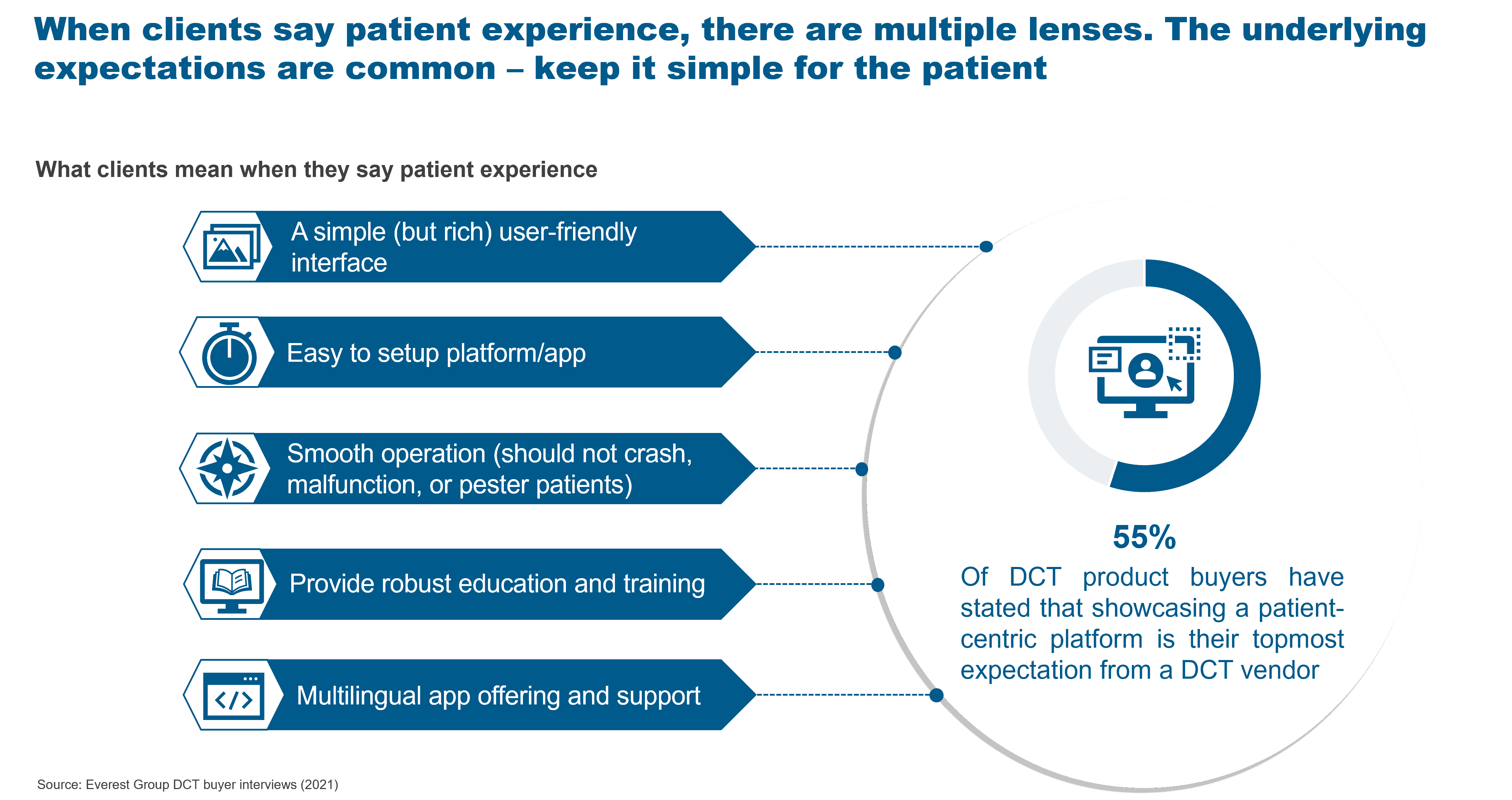
Five factors to enhance patient experience with DCTs
Patient experience can be broken down into the following aspects:
- User-friendly interface – The User Interface (UI) of DCT applications and devices must be simple, yet effective. They must provide clear instructions and display only relevant and concise content. It should be well organized, making all options easily accessible and ensuring that the application can be used with minimal explanation
- Easy to set up platform/app – Patients should have an easy time setting up a wearable, sensor, or application. It should be intuitive even to an average user with limited exposure to digital devices. The device should be as close as possible to a ready-to-use mode
- Smooth operation – The applications or devices should not pester patients with unnecessary notifications, malfunctions, or failures that would cause unwanted frustrations, resulting in reduced patient engagement. A smooth operation with minimal or zero disruption is the best-case scenario
- Robust education and training – Patients come with different levels of digital literacy, and they need to be supported during the trials. They must be aware of how to enroll themselves for the trial, schedule appointments, feed in data, and get important information about their health and the trial. Sponsors can create the knowledge pool, conduct training sessions, and build artificial intelligence (AI) bots to provide education and training to patients
- Multilingual app and support – To reach a global audience, multilingual offering and support must be available. The devices or applications used should provide instructions and information in the commonly used languages across the world. If a trial is geographically focused, the regional language should be configured in the device
Enterprises want DCT solutions to integrate smoothly into the daily lives and operations of patients. Patients should not feel isolated when doing the trial since the significant amount of digital literacy required might deter them from participating.
Vendors also need to be aware of the top patient-related challenges that might hinder them from elevating the patient experience through their products and services. Multiple challenges might lead to an inferior experience, resulting in disengagement and dropouts. DCT vendors and enterprise buyers must identify these challenges and take discrete steps to improve the patient experience and engagement.
Keep following this space as we dive into the top patient-related challenges and present initiatives aimed at improving the patient experience.
What are your views on the patient experience in DCTs? Reach out to [email protected] and [email protected] to discuss more.










