Blog
US Drug Pricing Disruption: Strategic Impact of the Most-Favored-Nation (MFN) Executive Order for Life Sciences

On May 12, 2025, President Donald Trump signed a high-impact executive order titled “Delivering Most-Favored-Nation Prescription Drug Pricing to American Patients.” The order directed the U.S. Department of Health and Human Services (HHS) to implement a pricing framework, whereby the U.S. pays no more for prescription drugs than the lowest price charged in peer Organisation for Economic Co-operation and Development (OECD) countries.
This Most-Favored-Nation (MFN) pricing model represents a significant escalation in federal efforts to contain healthcare costs, with projected price reductions ranging from 30% to 80% for select high-cost therapies.
The order also authorized the Food and Drug Administration (FDA) to expand drug importation programs and initiate regulatory actions intended to enforce compliance within a 30-day window. These measures signaled an ambitious, and potentially disruptive, shift in the U.S. pharmaceutical pricing framework, as we’ve highlighted below.
Reach out to discuss this topic in depth.
To illustrate the pricing disparities this executive order seeks to address, the exhibit below offers a comparative snapshot of five key therapies across the USA, UK, and Germany. The data highlights wide pricing gaps and identifies strategic risks tied to U.S. price convergence with OECD peers.
Table 1: International price disparities for select key therapies and strategic implications of the MFN order.
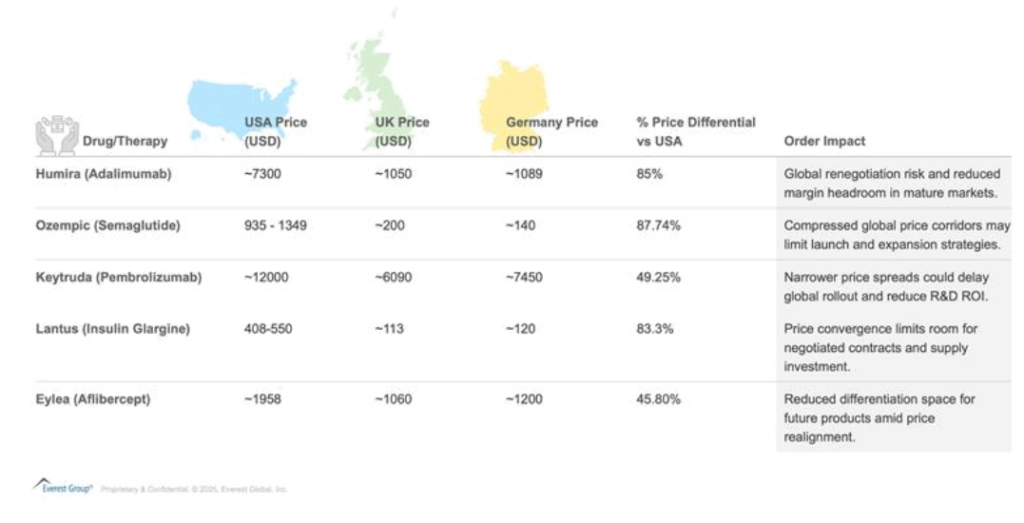
Note: Prices are triangulated from multiple sources and the average of prices are presented in the table
This directive has broad implications across the Healthcare and Life Sciences value chain. It places immediate pricing pressure on pharmaceutical manufacturers, while also targeting Pharmacy Benefit Managers (PBMs) such as CVS Caremark and Express Scripts for increased transparency.
The administration has argued that Pharmacy Benefit Managers (PBMs) contribute to inflated drug costs through opaque rebate structures and retained discounts. For U.S. consumers, who collectively spent over $800 billion on prescription drugs in 2024 (according to The Centers for Medicare & Medicaid Services (CMS) estimates), this policy signals potential financial relief, particularly for those relying on chronic therapies. However, the compressed 30-day compliance timeline, along with expected legal challenges, may delay full implementation or dilute its impact in the near term.
The MFN-based pricing framework poses a structural challenge for life sciences enterprises heavily reliant on margins in the USA. With the shift towards international price parity, the traditional model of subsidizing global Research and Development (R&D) through premium domestic pricing becoming less viable.
Enterprises may need to adjust pricing governance, reallocate revenue expectations, and reassess R&D funding strategies. Mid-sized and innovation-driven firms may face greater pressure too, while medical technology players linked to high-cost biologics could see intensified scrutiny around value and cost justification.
In response, companies are likely to revisit the global supply chain and cost structures to build resilience against pricing shocks. Financial planning will need to account for pricing volatility, especially in categories under payer focus. Increased investment in real-world evidence, health economics, and digital tools may also become essential to support reimbursement negotiations. Meanwhile, OECD payers observing the U.S. policy shift could adopt similar benchmarks, amplifying the need for globally coordinated market access strategies.
Implications to watch out for:
-
- Recalibration of global launch sequencing: Firms may delay or reorder product launches, prioritizing markets with higher net prices or more favorable Health Technology Assessment (HTA) environments, creating ripple effects on global access timelines
-
- Risk to portfolio planning and R&D strategy: With lower revenue potential in the USA, a strategic focus may shift from broad therapeutic portfolios to selective development of high-evidence, value-based assets
-
- Tighter controls on provider access and formularies: U.S. providers may face more restricted access to expensive therapies as payers and PBMs respond to pricing reforms with aggressive formulary tiering and cost controls
-
- Emergence of new contracting models: Value-based and indication-specific pricing frameworks are likely to gain traction as firms and payers seek to align reimbursement with clinical outcomes rather than fixed benchmarks
-
- Acceleration of generics and biosimilars adoption: The MFN policy is likely to boost adoption of generics and biosimilars in the USA, creating expanded opportunities for global manufacturers while raising the bar on regulatory compliance and quality assurance which may be further compounded by the patent cliff of blockbuster drugs
This executive order marks a turning point in the U.S. approach to pharmaceutical pricing, signaling a broader trend of pricing convergence and payer empowerment. While the order’s legal and operational path remains uncertain, the strategic message is clear: the days of wide international price differentials are numbered.
Life Sciences enterprises must prepare for a more globally synchronized pricing environment, one where success will depend on agile market access planning, cost discipline, and deeper alignment between innovation, value, and reimbursement.
Providers, too, must anticipate greater pressure to demonstrate treatment value, adjust prescribing patterns, and navigate evolving access frameworks. Those who adapt early will be best positioned to lead in this next chapter of global healthcare economics.
If you found this blog interesting, check out our Agentic Artificial Intelligence (AI): From Science Fiction To Life Sciences Disruption | Blog – Everest Group, which delves deeper into another topic regarding the life sciences sector.
To share your perspectives and to hear more about the latest advancements in the life sciences sector, please reach out to Sanket Anshuman ([email protected]), Vandana Sharma ([email protected]) and Bhavya Sharma ([email protected]).