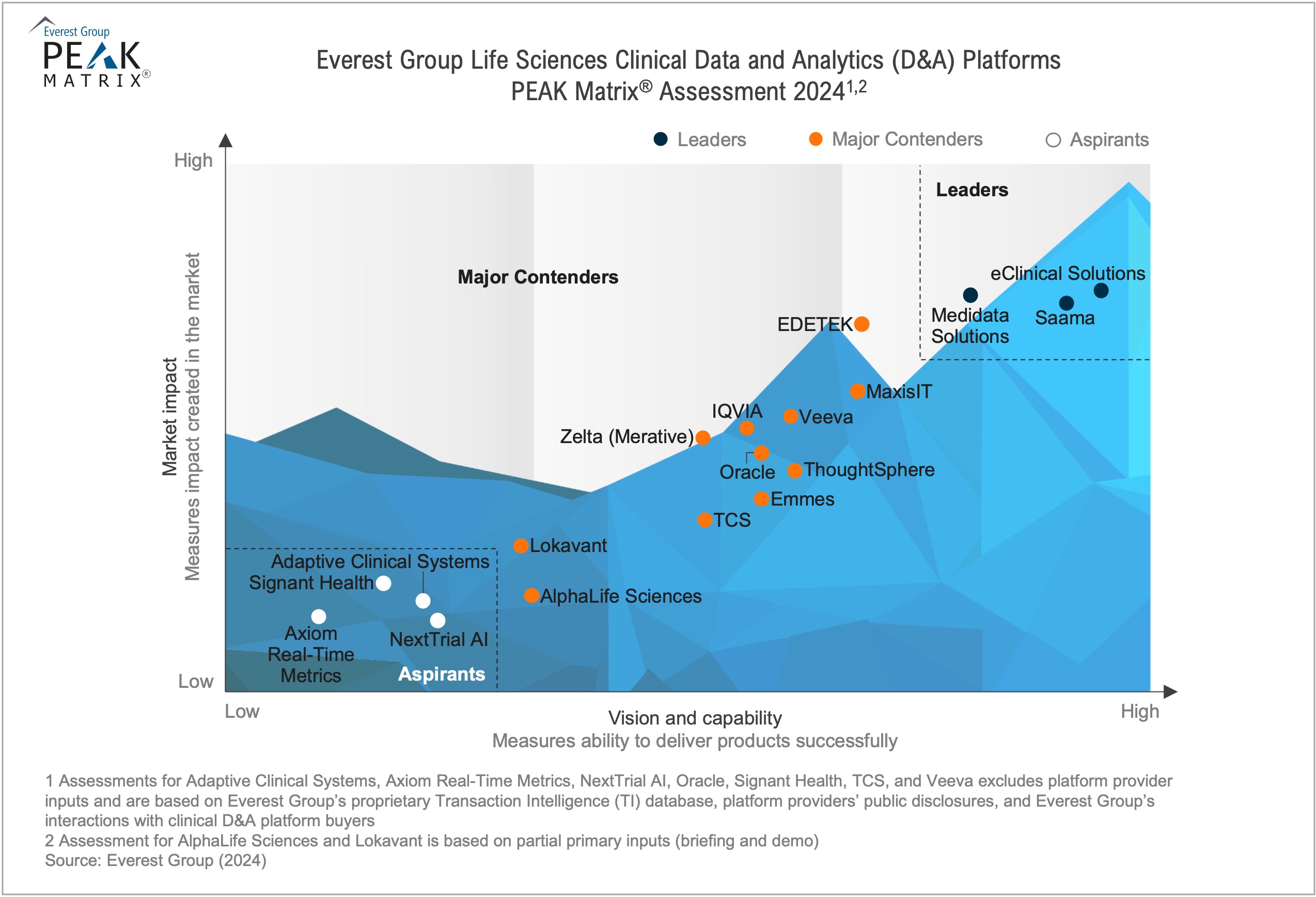
Life Sciences Clinical Data and Analytics (D&A) Platforms PEAK Matrix® Assessment 2024
Due to decentralized and hybrid designs, the growing clinical trial complexity generates vast data volumes from diverse sources and creates significant data management challenges. In response, sponsors are increasingly employing unified clinical data and analytics (D&A) platforms to centralize and standardize clinical data to gain actionable insights, offering real-time monitoring, predictive analytics, and risk management. These platforms transform disparate datasets into cohesive, structured formats, allowing stakeholders to detect outliers, predict adverse events, and generate on-demand dashboards and reports.
D&A platforms offer centralized repositories, Risk-Based Quality Management (RBQM), and seamless integration with Electronic Health Records (EHRs), wearables, and medical devices. These features improve data access and interoperability, contributing to more informed decision-making and enhanced quality and risk oversight. Clinical D&A platform providers are incorporating cutting-edge AI and generative AI capabilities to automate repetitive tasks, generate insights, and strengthen quality and risk management to address sponsor needs.
In this report, we analyze 18 clinical D&A platform providers featured on the Everest Group’s PEAK Matrix® based on their capabilities, offerings, and market impact. The report will empower buyers to choose the right provider for their sourcing considerations and enable providers to benchmark themselves against their competition.
This report is available to members.