Blog
The Changing Face of the CRO: Becoming the Everything Store for Decentralized Trials

On February 24th, 2021 we saw an announcement of one of the largest mergers/acquisitions that the CRO space has ever witnessed. ICON, the Dublin-based global CRO, announced that it has entered into a definitive agreement to acquire rival North Carolina-based PRA Health Sciences in a deal valued at US$12 billion. The deal, which makes the combined entity the second-largest CRO, next only to IQVIA (itself a merger of IMS Health and Quintiles), is one of the many instances of the rapidly consolidating CRO industry, accelerated by COVID-19.
Pushing the gas on decentralized trials
While there are a lot of potential synergies in this acquisition, such as minimum overlap in terms of geography, deeper therapeutic capabilities, and broader service offerings, one important takeaway from this acquisition echoed by Dr. Steve Cutler, Chief Executive Officer of ICON, is the shift towards Decentralized Trials (DCTs).
With decentralized trials gaining importance, thanks to the pandemic, there has been an increasing focus from CROs to shore up their capabilities and develop an integrated solution. For instance, Bioclinca and ERT recently announced a merger that enabled the combined entity to provide holistic solutions in eCOA, imaging, and clinical trial management solutions. ICON, through this acquisition, has set out to achieve an integrated offering in DCTs as well. It seeks to combine its home health services, site network, and wearables technology with the mobile health and connected health platforms and other real-world data solutions from PRA Health Sciences.
Becoming the everything store for decentralized trials
Traditionally, a CRO was considered a business process service provider, managing trial operations in regulatory, safety, and clinical conduct. Very few offered technology solutions along with business process services as this was often considered the forte of product vendors such as Oracle Health Sciences and Medidata. However, with the pandemic halting clinical trials, stakeholders analyzed how to restart paused clinical trials by virtualizing certain components of the trials through some short-term fixes, such as use of eConsent and eCOAs solutions, resulting in an uptick in DCTs.
Initially, partnerships had been the preferred route for CROs to support DCTs, for example, the Covance partnership with Medable wherein Covance’s patient and site interface would be powered by Medable’s DCT offerings. However, the recent M&A activity suggests that CROs are now considering adding product capabilities to enable DCTs by acquiring product players (such as what Bioclinica did with ERT) or by acquiring CROs with strong technology capabilities to support such trials (such as ICON and PRA Health Sciences). The result – offering a one-stop-shop solution to support DCTs, as highlighted in the visual below.
Figure 1: A one-stop shop solution for supporting decentralized trials
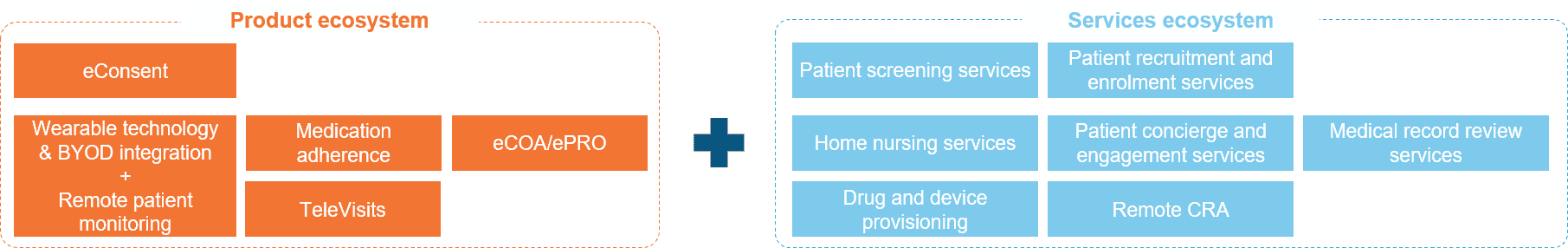
The advantage of such an integrated solution is that it augments the CRO’s value proposition to conduct DCTs – integrating platforms, services, site networks, and data capabilities, all into one place. Such CROs can now provide patient recruitment, engagement, and retention services (which has traditionally been their stronghold) using the underlying DCT suite through which the patient can enroll, record clinical outcomes, and engage in video consultation with the doctors/physicians. Additionally, they can also provide auxiliary support services, such as the provisioning of devices used for remote patient health monitoring and offering home nursing services aimed at reducing or eliminating patient visits to trial sites, medical record review services to check for completeness, accuracy, and compliance of medical data, and remote CRA services to oversee the DCT.
Implications for CROs
While, at first, the advantages of an in-house DCT suite seem to improve the value proposition for the CRO, it is also pertinent to note that in this scenario, CROs are also competing directly with DCT product vendors such as Medable and Science 37. The key challenge for CROs would be in convincing clients who still hesitate, while adopting technology offerings given their business process services heritage.
CROs aiming to walk down the acquisition path should keep the following pointers in mind:
- Innovate or perish: CROs would be competing directly with product vendors – an industry notorious for innovation. Investments aimed at improving the product quality, product enhancements, and fixing issues would be critical to win client trust
- Incorporate success stories: Showcasing client success stories and case studies will reduce client hesitation to adopt the one-stop DCT solution and drive increased product uptake
- Offer innovative commercial constructs: Traditional ways of contracting (for example, per study or volume-based constructs) may not work with DCTs. While offering clients a BPaaS construct, check for risk-sharing agreements as clients appreciate vendors who showcase skin in the game
Looking into the crystal ball
The DCT space is ripe for disruption and the string of M&A activities shows the increasing emphasis that CROs are putting on DCTs. As efforts to improve the value proposition intensity and innovation ensue, the industry can expect more tuck-in acquisitions and even some mega-mergers, such as ICON and PRA Health Sciences, to continue well into the future. What are your thoughts on this? Let us know at [email protected] and [email protected]