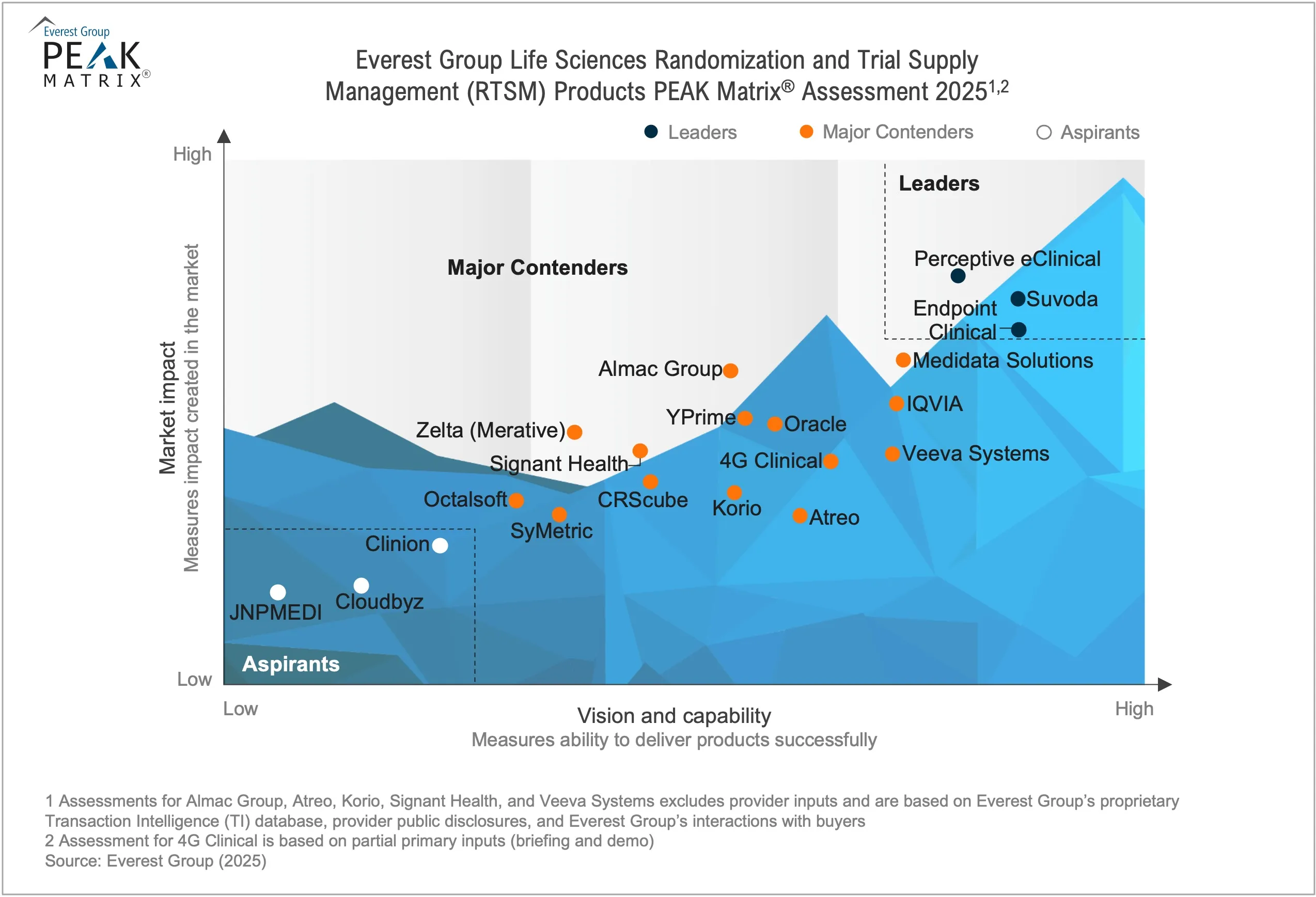
Life Sciences Randomization and Trial Supply Management (RTSM) Products PEAK Matrix® Assessment 2025
Modern RTSM platforms now serve as a single source of truth for randomization, supply chain management, and study oversight, offering real-time inventory visibility, automated shipment triggers, and seamless data integration across eClinical and logistics systems. Sponsors and Contract Research Organizations (CROs) are increasingly seeking RTSM providers that offer enhanced flexibility for mid-study amendments, predictive resupply capabilities, and low-code configuration to accelerate study deployment. To meet these evolving needs, RTSM providers are investing in AI-driven functionalities such as automated User Acceptance Testing (UAT), protocol parsing, virtual support assistants, and dynamic forecasting tools to reduce study timelines and improve accuracy.
In this report, Everest Group analyzes 20 RTSM product providers featured on the PEAK Matrix® for their market impact and vision and capabilities. It aims to empower buyers to identify the most suitable partners for their randomization and clinical supply management needs and enable providers to benchmark their strategic and technology maturity against peers.
This report is available to members.