Blog
The Race to Vaccinate against COVID-19: Five Ways Outsourcing Providers Can Supercharge the Pharma Industry
As the pharma industry chases the development of new COVID-19 vaccines with the peril of virus mutations looming and cases across the globe surging, outsourcing service providers can play vital roles in helping fast-track this essential mission. Read on to learn how critical partnerships with IT and BPO providers can help vaccine manufacturers in product development and more by providing technology tools, artificial intelligence, automation and analytics, talent and other needed expertise.
More than 100 years after the Spanish flu, the world is again fighting a pandemic. Since it began in March 2020, the COVID-19 pandemic is still impacting the global population in multiple ways with new waves, casualties, lockdowns, border restrictions, and pandemic appropriate behavior requirements.
Despite the availability of vaccines, the majority of countries are unable to fully vaccinate their population. While population size (especially in India and China) and vaccine hesitancy are well-known factors, a range of production and supply-related challenges have also contributed to these delays. Let’s explore what can be done to accelerate these efforts.
The global supply chain is as strong as its weakest link
In the wake of a global standstill in early to mid-2020, vaccine development was obstructed by the lack of appropriate raw materials, coordination gaps among multiple teams, lab-proofing and maintenance, and a manpower crunch among many other factors. Adding to the woes of biopharmaceutical manufacturers were challenges in carrying out clinical trials processes such as patient enrollment, site management and monitoring, and data management.
This led to study sites being either paused or slowed down. Although collaborations with Contract Research Organizations (CROs) and third-party vendors and rapid adoption of innovative practices (such as decentralized trials, telehealth, remote monitoring, etc.) ensured emergency approval of several vaccine candidates, the mammoth task of mass-production remains a critical challenge, hence, eclipsing the current success achieved so far.
The gigantic target of mass production of approved vaccines
The scarcity of highly specialized raw materials due to export restrictions has proved to be the biggest impediment to mass production. Moderna, one of the earliest producers of the COVID-19 vaccine, had to cut its shipments to Canada and the U.K. citing the volatile availability of human and material resources. Other pharma majors such as AstraZeneca and Pfizer observed similar restrictions. Pfizer, which sources 280 different materials from 19 countries for its mRNA vaccine, expressed its apprehensions in fulfilling supply commitments amidst future threats from increasing market competition as the voices for waiving IP protections grew stronger. Moreover, the availability of consumables and fill-finish elements such as vials, syringes, seals, single-use bags, sterile filters, etc. further stretched lead times.
While a few suppliers were able to ramp up their capacity, travel restrictions hampered vendors’ plans to install new equipment and commission new manufacturing lines. Additionally, the lack of adequate cold storage facilities, related equipment, insulated vans, trained personnel, and the absence of efficient network management systems caused further delays.
Vaccine delivery was further slowed by regulatory diversity across countries with each having its own guidelines and unavoidable compliances pertaining to Good Manufacturing Practices (GMPs) and quality standards. Let’s explore what can be done to accelerate these efforts.
Third-party support proves vital
Enterprises required third-party support in filing regulatory documents, ensuring data compliance, and capturing and reporting adverse events without comprising data quality or security. Responding to this need, IT and BPO players extended critical technology expertise to vaccine manufacturers and regulatory agencies.
Today, with the danger of virus mutations threatening and rising COVID-19 cases around the globe, pharma companies have embarked upon the onerous task of inventing new vaccines and scaling up production and distribution capacities. Service providers can (and have) fast-tracked the vaccine quest in more ways than one.
Here are five ways service providers can emerge as vital partners in the success of these undertakings:
- Location strategy: The initial optimism around vaccines suffered a blow when India faced its devastating second wave of COVID-19 and had to prioritize vaccines for its citizens. Similar vaccine protection controls were also deployed by the U.S. and the EU. This led some countries such as the UK to consider setting up facilities within their boundaries to reduce dependencies and avoid repercussions due to potential COVID-19 surges in their regions.
Service providers have the opportunity to deploy their expertise and help different stakeholders like governments and vaccine producers determine the ideal locations for setting up production facilities by evaluating strategic metrics such as availability of cold chains, assessing licensing requirements, talent accessibility, and procurement easiness.
- Vaccine development: Since no playbook exists for churning out a vaccine instantly every time a novel health crisis faces us, researchers run multiple iterations of experiments with different compounds to arrive at the combination triggering an immune response.
Artificial intelligence providers have a transformational role to play in enabling researchers to run simulations faster. DeepMind, a London-based AI company has leveraged its neural network, AlphaFold to simulate the structure of the SARS-CoV-2 coronavirus.
- Vaccine distribution: Ensuring vaccine distribution across a nation is a mammoth task. Service providers can leverage their technical expertise and develop vaccine distribution and administration dashboards to help governments keep track of their stocks, prevent stock-outs, and ensure the timely delivery of vaccines to achieve their vaccinations goals.
Infosys-owned Simplus has built a Salesforce-based vaccine management solution for the U.S. federal government and offers a wide spectrum of vaccine management services such as campaign management, citizen registration, prioritization, provider enrollment, supply chain visibility, forecasting, vaccine administration, and wellness surveys, and adverse event monitoring.
- Pharmacovigilance: With several COVID-19 vaccines receiving emergency-use authorization by different regions and countries, the entire spectrum of adverse effects of vaccines is still in the discovery phase. As time progresses, the number of data points collected will also increase, leading to the development of more robust AI systems that can predict Adverse Events Following Immunization (AEFI). This, in turn, will help manufactures improve the immunization outcomes.
Service providers have a critical role to play as technology enablers. For instance, Genpact’s PVAI solution has been deployed by the Medicines and Healthcare Products Regulatory Authority (MHRA) – the UK’s counterpart to the U.S. Food and Drug Administration – to intelligently track AEFIs.
- Ensuring talent availability: In one of the COVID-19 vaccine manufacturing facilities in the U.S., run by Emergent BioSolutions, millions of vaccine doses were contaminated due to unsanitary conditions and poorly trained staff. This incident demonstrates how ensuring skilled talent is a critical necessity for pharma companies, which is further strengthened by the fact that Pfizer is planning to set up a vaccine facility in Africa for its mRNA-based vaccine.
Service providers have an opportunity to extend their talent sourcing services for hiring and training skilled professionals who can hit the ground running as early as possible.
The opportunities for service provider-enterprises collaboration are ever-increasing
Analytics and automation, one of the few technology areas where enterprises generally lack expertise, is expected to gain tremendous traction as pharmas become more aware of the advantages of leveraging outsourcing providers’ capabilities. We predict two major developments to play crucial roles:
- The ‘novel’ vaccine development methodologies: While Pfizer-BioNtech’s vaccine is a novel mRNA-based vaccine, Cuban scientists have developed a promising vaccine candidate – Soberana 2, the only ‘conjugate COVID-19 vaccine’ that combines the virus’s receptor-binding domain with a deactivated form of tetanus to boost the immune response. Every novel technique and vaccine constituent paves the way for more methods of vaccine development against current and future diseases. This has the potential to open a plethora of opportunities for service providers to utilize their automation and analytics capabilities for a more robust design system.
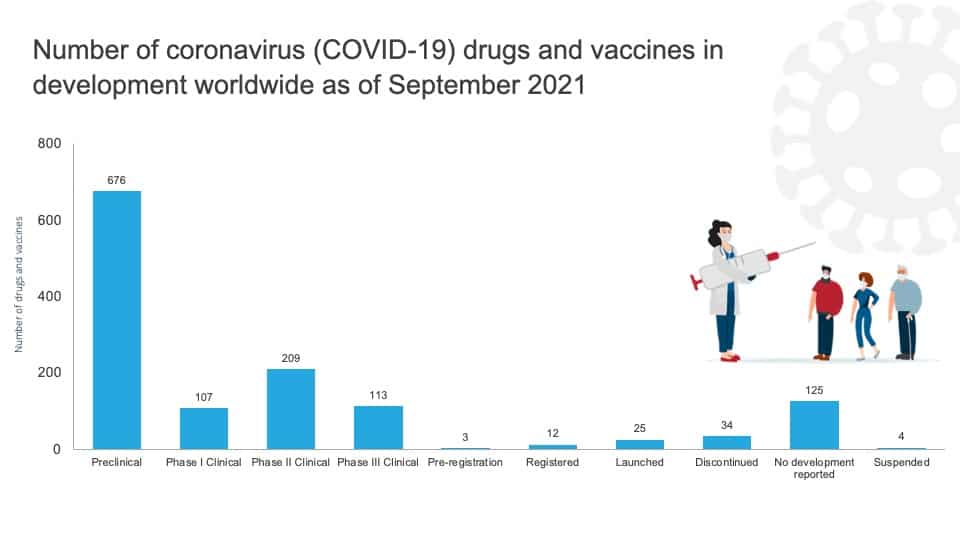
Number of coronavirus (COVID-19) drugs and vaccines in development worldwide as of September 2021, by phase (Source: https://pharmaintelligence.informa.com/)
- Vaccine alliances: With new virus mutations and some countries insisting on a third booster shot for their citizens, vaccine alliances like COVID-19 Vaccines Global Access (COVAX) and GAVI will likely continue playing larger roles in the future. A major responsibility of such alliances includes equitable distribution of vaccines throughout the world. Hence, they can leverage tools provided by service providers to process vital data and act on decisions that promise the quickest success rate.
Beyond the current COVID crisis, pharmaceutical enterprises and outsourcing service providers are partnering to transform the landscape in many ways from clinical trials to pharmacovigilance and Quality Risk Assessments (QRA). For more details, check out the latest research reports published by Everest Group here and reach out to [email protected] or [email protected] [email protected].